43 label each carbon atom with the appropriate geometry
Post Lecture Lewis Structures, Problem Set #6 (Ch 5), Problem ... - Quizlet Draw an appropriate Lewis structure for CH2CHCH3. Identify the molecular geometry around each carbon atom in CH2CHCH3 using VSEPR theory. specify whether the molecule CH2CHCH3 is polar or nonpolar and explain why. Identify the hybridization of all interior atoms for the molecule CH2CHCH3, according to valence bond theory, in the diagram showing ... OChem Spring 2017 Exam 1 Flashcards - Quizlet 6 carbons? hexane Draw kekule structure of Methane Draw the kekule structure of Butane Draw the line structure of Butane Carbon must have _____ bonds 4 Draw the kekule structure of CH₃CHClCH₃ Draw the line structure of CH₃CHClCH₃ (2 configurations) Draw the kekule structure for CH₃CH (CH₃)CH₂CH₃ Name structure (cover right side of screen) Methane
Label each carbon atom with the appropriate geometry. Label each carbon atom with the appropriate geometry. Two binary symmetric channels (BSC) are connected in cascade as shown below. input — BSC BSC 2 output 1 Both the channels have the same transition probability and the error/cross over probability... Posted one year ago Q:
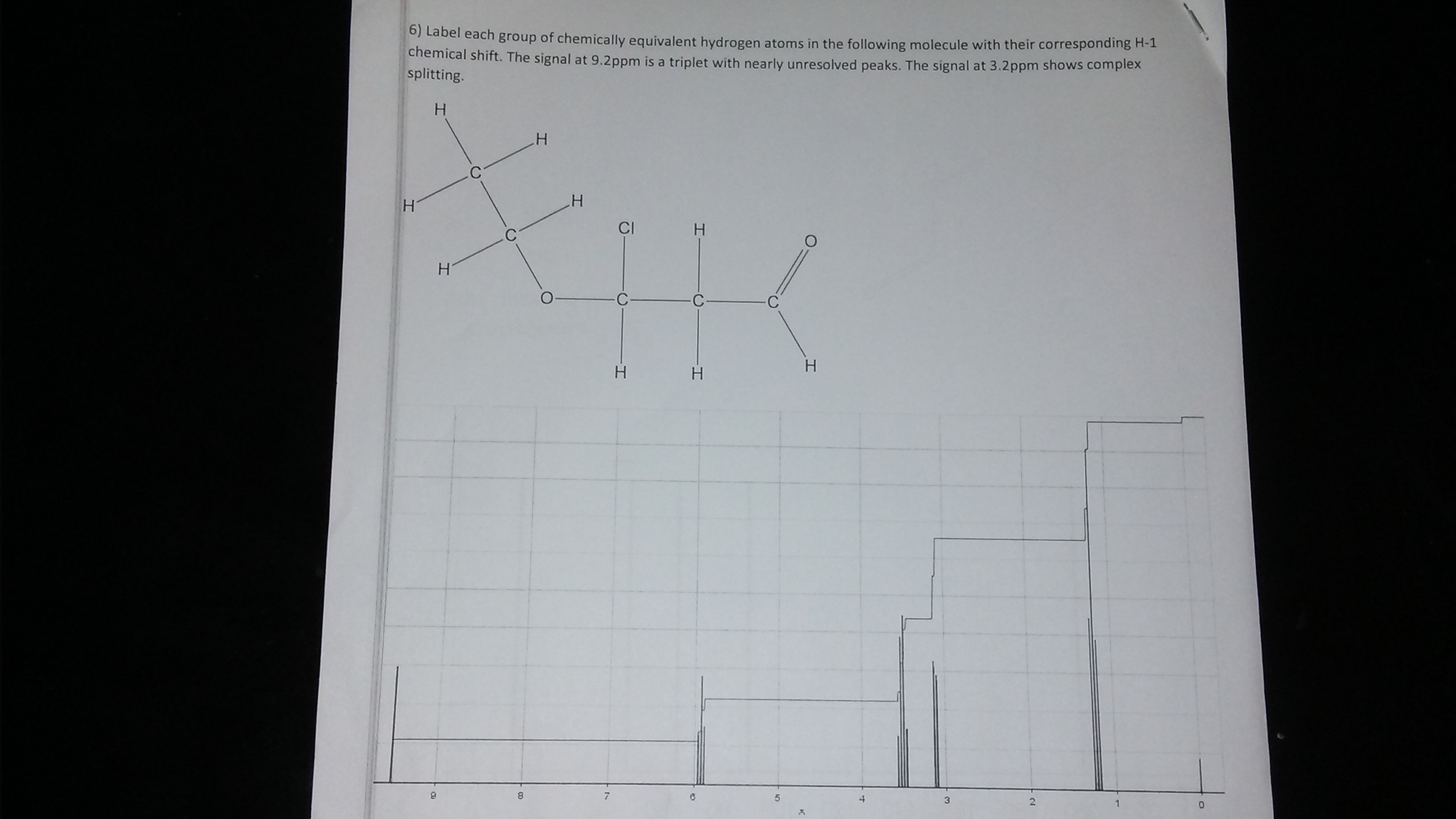
Label each carbon atom with the appropriate geometry
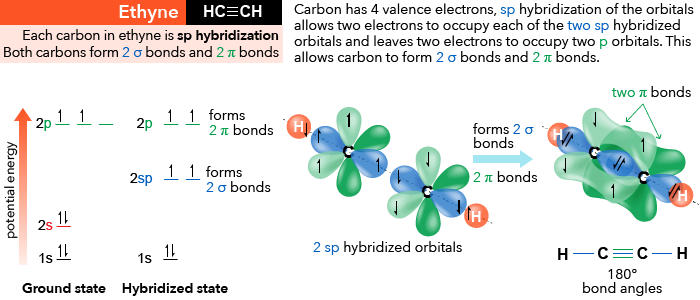
Label each carbon atom with the appropriate geometry. CH2CH(CH2)2CCH The hybrid orbitals of carbon involve in bond formation with hydrogen. Hence, the geometry at each carbon depends on the type of hybridization. Fundamentals The geometry of sp3 hybridized carbon atom is tetrahedral. The geometry of sp2 the hybridized carbon atom is trigonal planar. The geometry of sp hybridized carbon atom is linear. Label each carbon atom with the appropriate geometry. Label each carbon atom with the appropriate geometry. trigonal planar CH2 trigonal planar CH trigonal planar linear tetrahedral ČH2 tetrahedral bent tetrahedral ČH2 trigonal pyramidal linear bent CH ↑ ↑ ↑ ↑ Related Book For Free Organic Chemistry 8th edition Authors: L. G. Wade Jr. Get help fromAccounting Tutors ⚗️Label each carbon atom with the appropriate geometry. Bin 1 points to ... A single C-C or C-H bond is in a tetrahedral geometry, the carbon atom is bonded to four species with a bond angle of 109°. A C=C bond is trigonal planar with a bond angle of 120°. Lastly, a C≡C bond has a linear geometry with a bond angle of 180° between the atoms of the bond. Survey Did this page answer your question? Not at all Slightly Kinda
Label each carbon atom with the appropriate geometry. Mastering Chemistry Tro Chapter 10 Flashcards - Quizlet Determine the molecular geometry about each interior atom and make a sketch: C2H6 (skeletal structure: H3CCH3) ... it would be appropriate to label hydrogen with the symbol ____ and oxygen with the symbol ____. ... According to the electronegativity difference between the atoms in carbon dioxide, it would be appropriate to label oxygen with the ... What is the geometry around each of the three central atoms in the CH ... Explanation: We must first draw the Lewis structure of acetic acid. (Adapted from Chemistry@TutorVista.com) Carbon 1 This atom has four atoms directly attached and no lone pairs. Its electron geometry and its molecular geometry are both tetrahedral as in methane. Carbon 2 This atom has three atoms directly attached and no lone pairs. Label each carbon atom with the appropriate geometry. Label each carbon atom with the appropriate hybridization. sp,sp2 or sp3 Label each carbon atom with the appropriate hybridization. sp,sp2 or sp3 Posted one year ago Recent Questions in Chemistry Name the geometry around each carbon atom. What is the hybridization of ... Below is the Lewis structure of cyclohexane (C 6 H 12) molecule, a cyclic compound used in the manufacture of nylon and found in the distillation of petroleum.. Name the geometry around each carbon atom. What is the hybridization of each carbon atom?
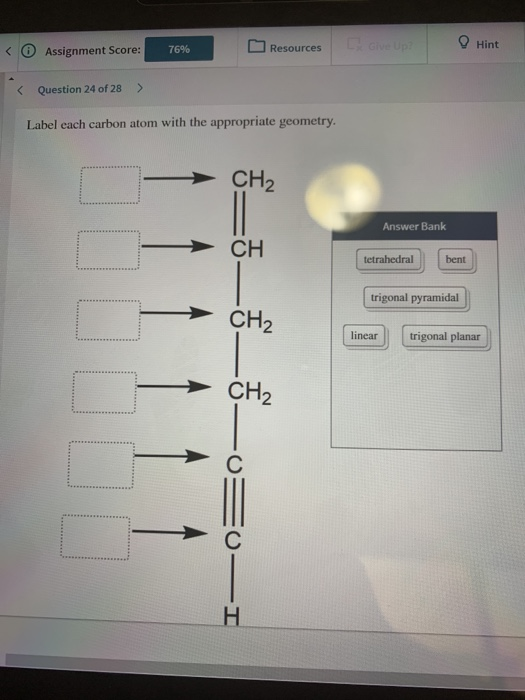
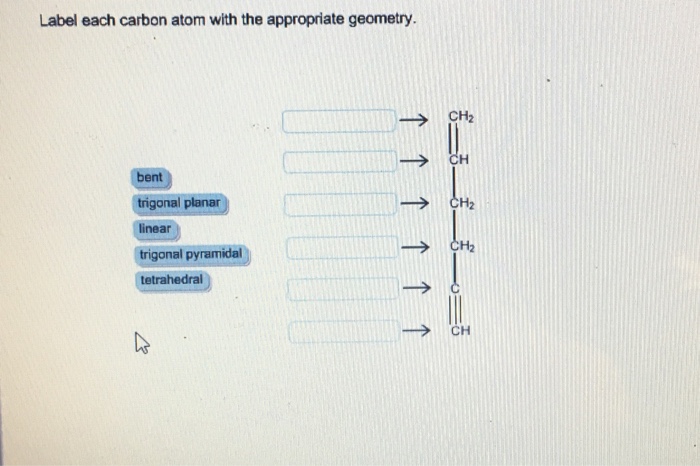
label each carbon atom with the appropriate hybridization 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One... Final Exam Flashcards | Quizlet Sort the graphical representations with the correct categories of geometry. flat- sheet open- saddle shaped closed- spherical. Label each shape with the correct value - omega mass >1 - omega mass <1 - omega mass=1 ... create a timeline that traces the full history of a proton that came to be part of the earth, say, in the nucleus of a carbon ... Label each carbon atom with the appropriate geometry. - OneClass 5 Nov 2019 Label each carbon atom with the appropriate geometry. Trigonal pyrimidal Trigonal planar Tetrahedral Linear Bent CH2 (double bond) CH (single bond) CH2 (single bond) CH2 (singlebond) C (triple bond) CH Show full question Answer + 20 Watch For unlimited access to Homework Help, a Homework+ subscription is required. Bunny Greenfelder Lv2 (Get Answer) - Label each carbon atom with its optimum C-C-C bond angle ... Label each carbon atom with its optimum C-C-C bond angle. 180 degree 109.5 degree 120 degree 90 degree. ... Predict the geometry of each interior atom in acetic acid Drag the appropriate labels to their respective targets. Note: not all labels will be used. ... The molecular geometry of water is best described as: A) Pyramidal B) Linear C) Bent ...
⚗️Label each carbon atom with the appropriate geometry. Bin 1 points to ... A single C-C or C-H bond is in a tetrahedral geometry, the carbon atom is bonded to four species with a bond angle of 109°. A C=C bond is trigonal planar with a bond angle of 120°. Lastly, a C≡C bond has a linear geometry with a bond angle of 180° between the atoms of the bond. Survey Did this page answer your question? Not at all Slightly Kinda Label each carbon atom with the appropriate geometry. Label each carbon atom with the appropriate geometry. trigonal planar CH2 trigonal planar CH trigonal planar linear tetrahedral ČH2 tetrahedral bent tetrahedral ČH2 trigonal pyramidal linear bent CH ↑ ↑ ↑ ↑ Related Book For Free Organic Chemistry 8th edition Authors: L. G. Wade Jr. Get help fromAccounting Tutors Label each carbon atom with the appropriate geometry. CH2CH(CH2)2CCH The hybrid orbitals of carbon involve in bond formation with hydrogen. Hence, the geometry at each carbon depends on the type of hybridization. Fundamentals The geometry of sp3 hybridized carbon atom is tetrahedral. The geometry of sp2 the hybridized carbon atom is trigonal planar. The geometry of sp hybridized carbon atom is linear.


Post a Comment for "43 label each carbon atom with the appropriate geometry"