› view › bias-patient-reportedBias for Patient-Reported Outcomes in Open-Label Cancer ... Feb 28, 2019 · A common concern with patient-reported outcomes (PROs) in open-label trials is that a patient’s knowledge of treatment received could influence their view and reporting of their symptoms. With this in mind, members of the US Food and Drug Administration explored the possibility of such bias in a recent viewpoint published in JAMA Oncology. “In light of the anticipated growth in PRO submissions to the FDA,” the authors reasoned, “we outline our thoughts on the ways in which open label ...

Open-label study bias

23 July 2022 AperTO - Archivio Istituzionale Open Access dell ...

Randomized controlled trial - Wikipedia

Bias was reduced in an open-label trial through the removal ...
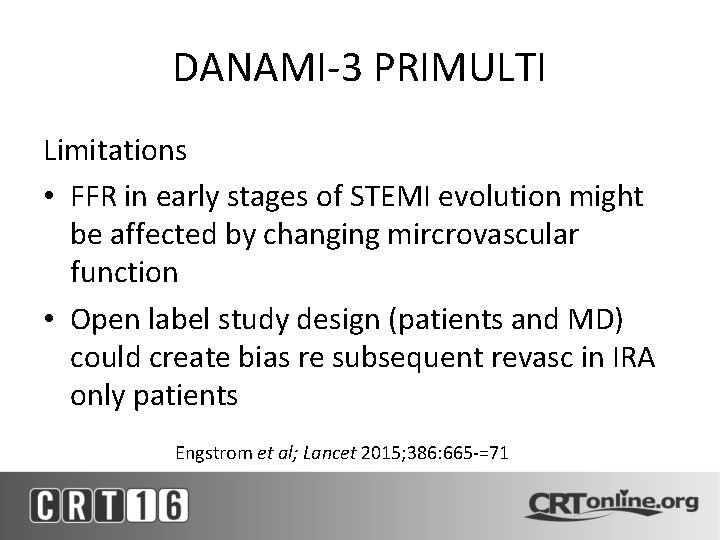
Management of MultiVessel Disease in the STEMI Patient

Design characteristics, risk of bias, and reporting of ...

Open-Label Placebo Treatment: Outcome Expectations and ...
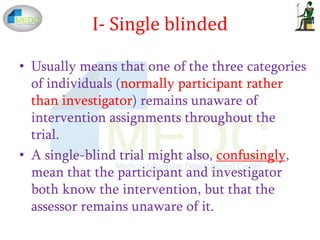
Blinding in clinical trilas

Effect of Fibrates on Lipid Profiles and Cardiovascular ...
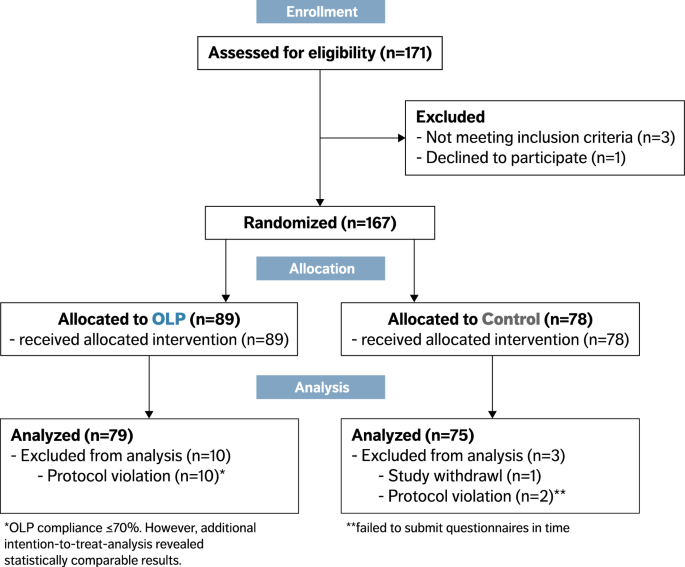
Effects of open-label placebos on test performance and ...

3.clinical trials

Potential sources of bias in included studies Study Possible ...

Revised Cochrane risk of bias tool for randomized trials (RoB ...

Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct ...

Understanding Data Bias. Types and sources of data bias | by ...

Header Biostatistics and Epidemiology Lillian Sung MD Ph

Effect of training traditional birth attendants on neonatal ...

Providing open-label placebos remotely—A randomized ...
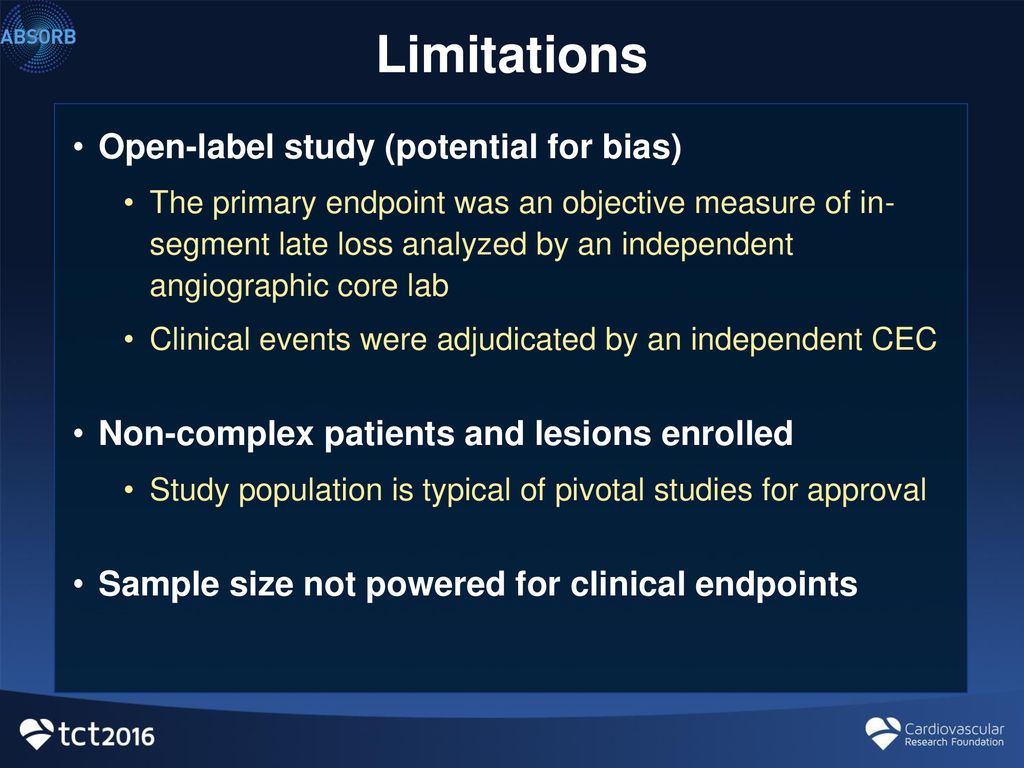
Runlin Gao, M.D. On behalf of ABSORB China Investigators ...

The Efficacy and Safety of the Application of Pulsed ...
![PDF] Clinical trial methodology to assess the efficacy ...](https://d3i71xaburhd42.cloudfront.net/a00f5af9933a8f9c61da678199905782adf4210d/8-Table4-1.png)
PDF] Clinical trial methodology to assess the efficacy ...

Supplemental Content 2. Qualitative analysis through Cochrane ...

The concept of blinding in clinical trials

Biometrics India, Pfizer Global R & D - ppt video online download

Epidemiology Clinical Trials Outline Basic principles Biases ...

Mohsin Furqan on Twitter: "1/3 HOST-EXAM Trial ❓Efficacy and ...

ESC 365 - Optimal dual antiplatelet treatment (DAPT) duration ...
![PDF] Bias was reduced in an open-label trial through the ...](https://d3i71xaburhd42.cloudfront.net/814ad6ccbfa9defca4d2b00c4672f9070cf6b8da/16-Figure1-1.png)
PDF] Bias was reduced in an open-label trial through the ...

PDF) Reducing bias in open-label trials where blinded outcome ...

HETEROGENEITY AND SOURCES OF BIAS Olaf DekkersSnderborg2016 ...

Clinical Trial Results 2020: The Oncologist

About the 4WHIM Trial | 4WHIM Clinical Research Study

Epidemiology and Clinical Research Design, Part 1: Study ...

Bias for Patient-Reported Outcomes in Open-Label Cancer ...

Targeting tyrosine- kinase receptors: pitfalls and benefits ...

Efficacy and safety of hydroxychloroquine as pre-and post ...
![PDF] The Clinical Viewpoint: Definitions, Limitations of ...](https://d3i71xaburhd42.cloudfront.net/0d341492d445073e64d1ccb6985e4ad763a3d1f7/2-Table1-1.png)
PDF] The Clinical Viewpoint: Definitions, Limitations of ...

Oral fexinidazole for stage 1 or early stage 2 African ...
PDF) What is an open label trial?













![PDF] Clinical trial methodology to assess the efficacy ...](https://d3i71xaburhd42.cloudfront.net/a00f5af9933a8f9c61da678199905782adf4210d/8-Table4-1.png)




![PDF] Bias was reduced in an open-label trial through the ...](https://d3i71xaburhd42.cloudfront.net/814ad6ccbfa9defca4d2b00c4672f9070cf6b8da/16-Figure1-1.png)








![PDF] The Clinical Viewpoint: Definitions, Limitations of ...](https://d3i71xaburhd42.cloudfront.net/0d341492d445073e64d1ccb6985e4ad763a3d1f7/2-Table1-1.png)


Post a Comment for "38 open-label study bias"