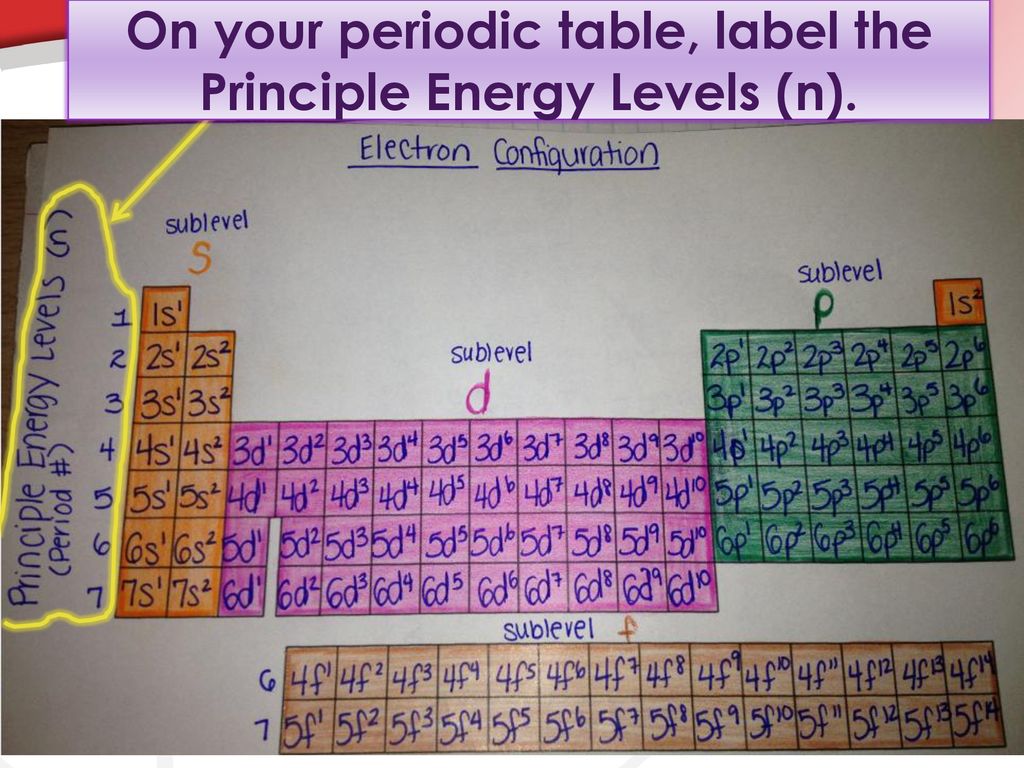
45 what term is used to label the energy levels of electrons
What is the term used to label the energy levels of electron What is the term used to label the energy levels of electron 1 See answer Advertisement Greatanonymous09 Answer: The quantum mechanical model of the atom estimates the probability of finding an electron in a certain position. ... Circle the letter of the formula for the maximum number of electrons that can accupy a principal energy level. What term is used to label the energy levels of electrons? - Answers What term is used to label energy levels of electrons? Principal quantum numbers (n). What is the term valance band? It refers to the energy levels in an atom where the electrons that participate...
Electrons in an Atom Flashcards | Quizlet used to label energy levels of electrons. s. used to denote a spherical orbital. top of p is on right side. px. top of p is on left side. py. p is straight. pz. ... Sets with similar terms. Chemistry Chapter 4. 21 terms. athenanolan. Chemistry Chapter 4. 21 terms. lanashanab. Chem chap 4. 65 terms. Matt_Stasolla24. Chapter 5 - Electrons in Atoms.

What term is used to label the energy levels of electrons
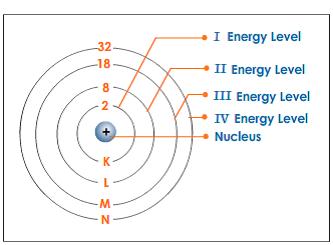
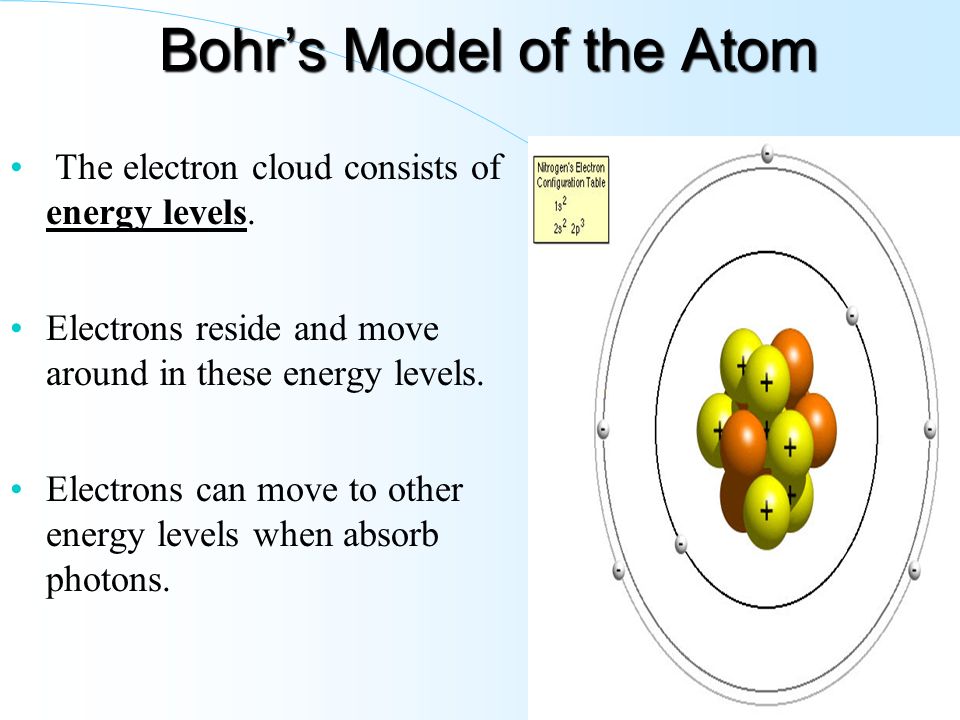
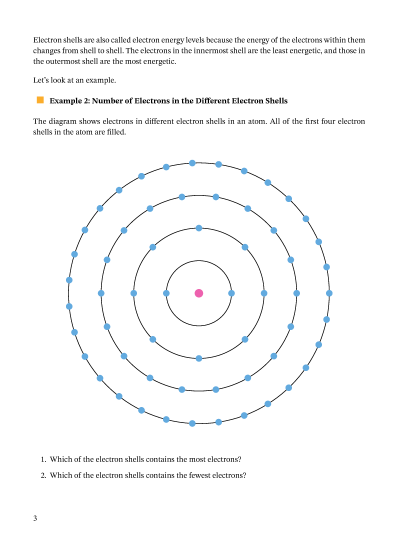
Energy Level Diagram - Different Energy Shells Around the Nucleus - BYJUS What is an energy level diagram? In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. Bohr developed this model of the atom which says the electrons revolve around the nucleus in a circular path called an orbit. What term is used to label the energy levels of electrons? - Brainly.com kiki6539 The term that is used to label the energy levels of electrons are principle quantum numbers and a valance band refers to the "energy levels in an atom where the electrons that participate in bonding occupy. These energy levels correspond to those of the s and p orbitals of the outermost shell of the atom being considered." Hope this helps! Energy level - Wikipedia The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be quantized .
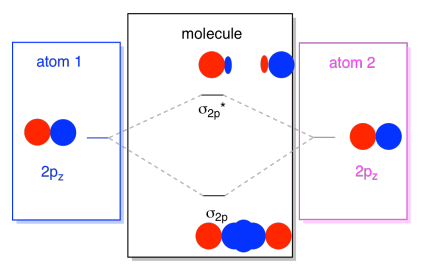
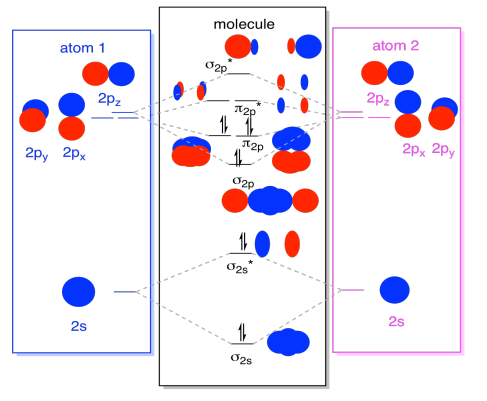
What term is used to label the energy levels of electrons. Energy levels | Article about Energy levels by The Free Dictionary In X-ray spectroscopy the rydberg (Ry) is used as the unit of energy; 1 Ry = 13.606 eV. In optical spectroscopy we often speak of the spectral term, which means the value of T = -ℰ/ hc. For atoms, T is reckoned from the ionization limit and is given in cm -1. M. A. E L'IASHEVICH Solved Molecule of study: [V(H20)] a) Write down the IUPAC - Chegg molecule of study: [v (h20)] a) write down the iupac name, the oxidation state and electron configuration of the transition metal. [3 marks) b) draw the molecular orbital (mo) diagram of the coordination complex to represent the sigma bonding. [5 marks) c) use orbital and symmetry symbols to label energy levels and assign electrons to the energy … Energy level | Article about energy level by The Free Dictionary In X-ray spectroscopy the rydberg (Ry) is used as the unit of energy; 1 Ry = 13.606 eV. In optical spectroscopy we often speak of the spectral term, which means the value of T = -ℰ/ hc. For atoms, T is reckoned from the ionization limit and is given in cm -1. M. A. E L'IASHEVICH chem - Name:_ Date _ Period_ FA-Electron Energy Levels... View chem from CHEM 115 at Georgetown University. Name:_ Date _ Period_ FA-Electron Energy Levels Background Electrons are organized within an atom; they each "live" in a specific place within
What is the term used to label the energy levels of electrons ... - Answers What term is used to label energy levels of electrons? Principal quantum numbers (n). What is the term valance band? It refers to the energy levels in an atom where the electrons that participate... How to Represent Electrons in an Energy Level Diagram You look on the periodic table and find that oxygen is atomic number 8. This number means that oxygen has 8 protons in its nucleus and 8 electrons. So you put 8 electrons into your energy level diagram. You can represent electrons as arrows. If two electrons end up in the same orbital, one arrow faces up and the other faces down. Solved Molecule of study: K3[Co(C2O4)3] a) Write down the - Chegg c) Use orbital and symmetry symbols to label energy levels and assign electrons to the energy levels in your diagram, considering that this compound is paramagnetic. [5 marks] d) Clearly identify the Δo energy splitting transition on your MO diagram and explain your choice. Calculate the splitting energy in cm-1 knowing that λmax = 600 nm. [5 ... Chapter 5 test Section 5.1 Flashcards | Quizlet The term that is used to label the energy levels of electrons is? S What letter is used to denote a spherical orbital? Dumbell All "p" orbital's are _____ shaped? 2n^2 What is the formula for the maximum number of electrons that can occupy a principal energy level? (Use "n" for the principal quantum number) 2,826 explanations 3,486 explanations
Energy level - Wikipedia The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be quantized . What term is used to label the energy levels of electrons? - Brainly.com kiki6539 The term that is used to label the energy levels of electrons are principle quantum numbers and a valance band refers to the "energy levels in an atom where the electrons that participate in bonding occupy. These energy levels correspond to those of the s and p orbitals of the outermost shell of the atom being considered." Hope this helps! Energy Level Diagram - Different Energy Shells Around the Nucleus - BYJUS What is an energy level diagram? In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. Bohr developed this model of the atom which says the electrons revolve around the nucleus in a circular path called an orbit.




:max_bytes(150000):strip_icc()/energylevels-56a129545f9b58b7d0bc9f39-5aeb7f1aae9ab800373981a3.png)




















Post a Comment for "45 what term is used to label the energy levels of electrons"