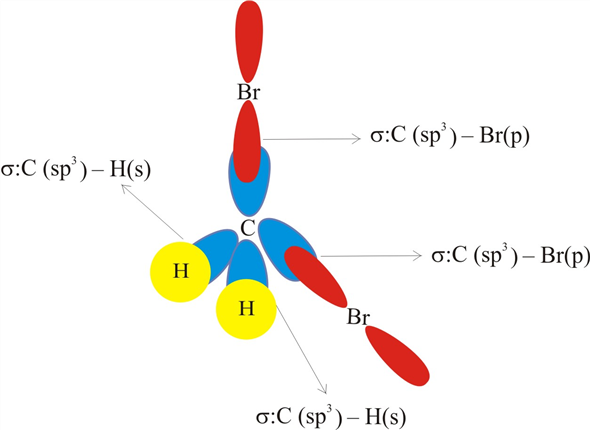
41 label all bonds in ch2br2 .
Solved In the sketch of the structure of CH Br2 label all - Chegg In the sketch of the structure of CH Br2 label all bonds. Drag the appropriate labels to their respective targets. CHEM 1210 Flashcards | Quizlet Now put all the given values in the ideal gas equation, we get: and, where, = pressure of gas = 374 mmHg = 0.492 atm (1 atm = 760 mmHg) V = volume of gas = 1.65 L T = temperature of gas = 305 K = number of moles of gas = ? R = gas constant = 0.0821 L.atm/mol.K Now put all the given values in the ideal gas equation, we get:
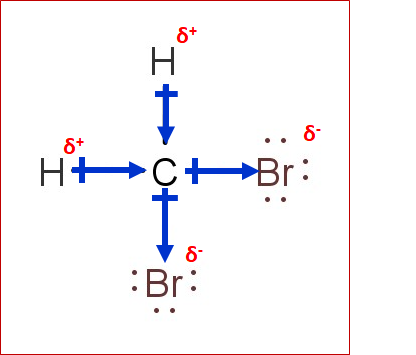
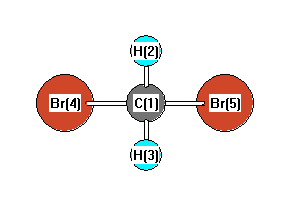
CH2Br2 Lewis Structure, Geometry, Hybridization, and Polarity In CH 2 Br 2, there are two types of bonds, ' C-H' and 'C-Br.' According to the electronegativity scale of Linus Pauling- • Electronegativity of C= 2.55 • Electronegativity of H=2.2 • Electronegativity of Br=2.96 The difference for 'C-H' and 'C-Br' comes out to be 0.35 and 0.41, respectively.

Label all bonds in ch2br2 .
The The Identify In Ch2br2 Hybridization C Atom Of [LCDI5V] If the C atom has 4 sigma bonds , as is the case in CH₂Br₂, there are 4 hybridized orbitals required, so 1 "s" orbital and 3 "p" orbitals hybridize to form an sp3 hybrid orbital So when asked to describe the shape of a molecule we must respond with a molecular The CH2Br2 molecule hybridization is four Solved Part B Label all bonds in CH2Br2 ... SOLVED:Write a hybridization and bonding scheme for each molecule ... before determining the hybridization and the orbital overlaps. To describe the bonds, we first need to draw the lewis structures. The first one is for C. H two, BR two With a total of 20 valence electrons that allows us to bond everything to carbon and then have enough to give the pro means and octet. Yeah. So, I should probably add in the octet here, mm hmm. SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CH2Br2 b. SO2 c. NF3 d. BF3. Answer (a) See solution (b) See solution (c) See solution (d) See solution. View Answer. Related Courses. Chemistry 101.
Label all bonds in ch2br2 .. Solved Part B Label all bonds in CH2Br2 Label the diagram by | Chegg.com Transcribed image text: Part B Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used H (p) H (p) Br (o) Br (p) Previous question Next question Solved Label all bonds in CH2Br2. Label all bonds in | Chegg.com Best Answer 92% (64 ratings) Transcribed image text: Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C (sp) H (s) o C (sp') Br (s) C (p) H (p) C (p) Br (p) C (sp) H (p) o C (sps) Br (p) C (sps) Br (p) reset help Previous question Next question OneClass: Label all bonds in CH2Br2? Get the detailed answer: Label all bonds in CH2Br2? ... Label all bonds in CH 2 Br 2? Answer +20. Watch. 1. answer. 2. watching. 668. views. For unlimited access to Homework Help, a Homework+ subscription is required. Kottherva Sreevidya Lv10. 5 Jan 2021. Unlock all answers. Get 1 ... How many bonds are in CH2Br2? - Answers No, hydrogen bonding only occurs in compounds where hydrogen (H) is bonded to nitrogen (N), oxygen (O) or fluorine (F). How many isomers of ch2br2? This compound (dibromomethane) has only one...
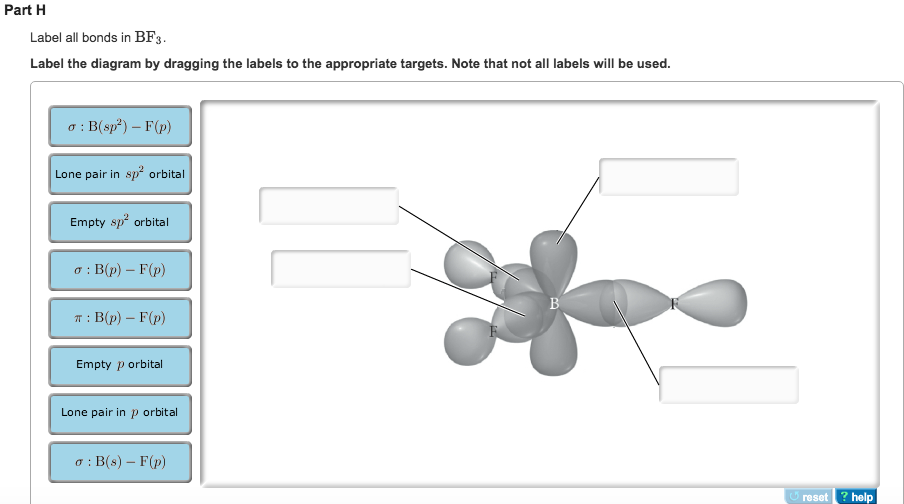
(Get Answer) - Label All Bonds In BF3. Label The Diagram By Dragging ... Label All Bonds In BF3. Label The Diagram By Dragging The Labels To The Appropriate Targets. Note That Not All Labels Will Be Used. Σ : B(S)-F(P) Empty P Orbital Lone Pair In Sp Orbital Σ : B(Sp2)-F(P) Σ : B(P)-F(P) Lone Pair In P Orbital Empty Sp2 Orbital Π : B(P)-F(P) Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in NF3. Label all bonds in BF3. Answer The molecule's name is CH2Br Br Br br dibromomethane. The molecule can be described as a derivative methane. The central atom of carbon is bonded with two hydrogen atoms, and two bromine. All bonds are sigma. CHEM: Chapter 10 Flashcards | Quizlet The sp3 and sp3d2 hybridization schemes have no unhybridized p-orbitals left to form π-bonds. Valence Bond Theory 62. Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2 c. NF3 d. BF3 Answered: Write a hybridization and bonding… | bartleby Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2 c. NF3 d. BF3 Question Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7.
Chapter 6, Chemical Bonding II Video Solutions, Chemistry: Structure ... Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. N2H2 (skeletal structure HNNH) b. Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C (sp) H (s) o C (sp') Br (s) C (p) H (p) C (p) Br (p) C (sp) H (p) o C (sps) Br (p) C (sps) Br (p) reset help Expert's Answer Solution.pdf Next Previous Posted 6 months ago Answered: In the sketch of the structure of… | bartleby Transcribed Image Text: In the sketch of the structure of CH2Br2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. (Solved) : Label Bonds Ch2br2 Label Bonds So2 Label Bonds Nf3 Label ... Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C(sp) H(s) o C(sp') Br(s) C(p) H(p) C(p) Br(p) C(sp) H(p) o C(sps) Br (p) C(sps) Br (p) reset help Show transcribed image text Expert Answer
SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CH2Br2 b. SO2 c. NF3 d. BF3. Answer (a) See solution (b) See solution (c) See solution (d) See solution. View Answer. Related Courses. Chemistry 101.
SOLVED:Write a hybridization and bonding scheme for each molecule ... before determining the hybridization and the orbital overlaps. To describe the bonds, we first need to draw the lewis structures. The first one is for C. H two, BR two With a total of 20 valence electrons that allows us to bond everything to carbon and then have enough to give the pro means and octet. Yeah. So, I should probably add in the octet here, mm hmm.
The The Identify In Ch2br2 Hybridization C Atom Of [LCDI5V] If the C atom has 4 sigma bonds , as is the case in CH₂Br₂, there are 4 hybridized orbitals required, so 1 "s" orbital and 3 "p" orbitals hybridize to form an sp3 hybrid orbital So when asked to describe the shape of a molecule we must respond with a molecular The CH2Br2 molecule hybridization is four Solved Part B Label all bonds in CH2Br2 ...

























Post a Comment for "41 label all bonds in ch2br2 ."