39 secondary label requirements
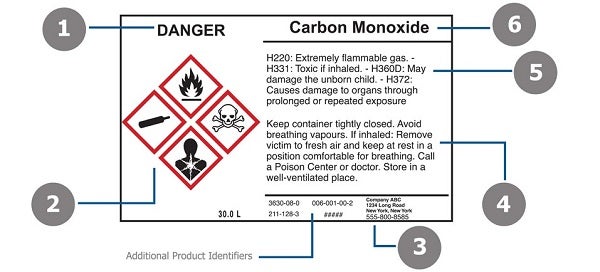
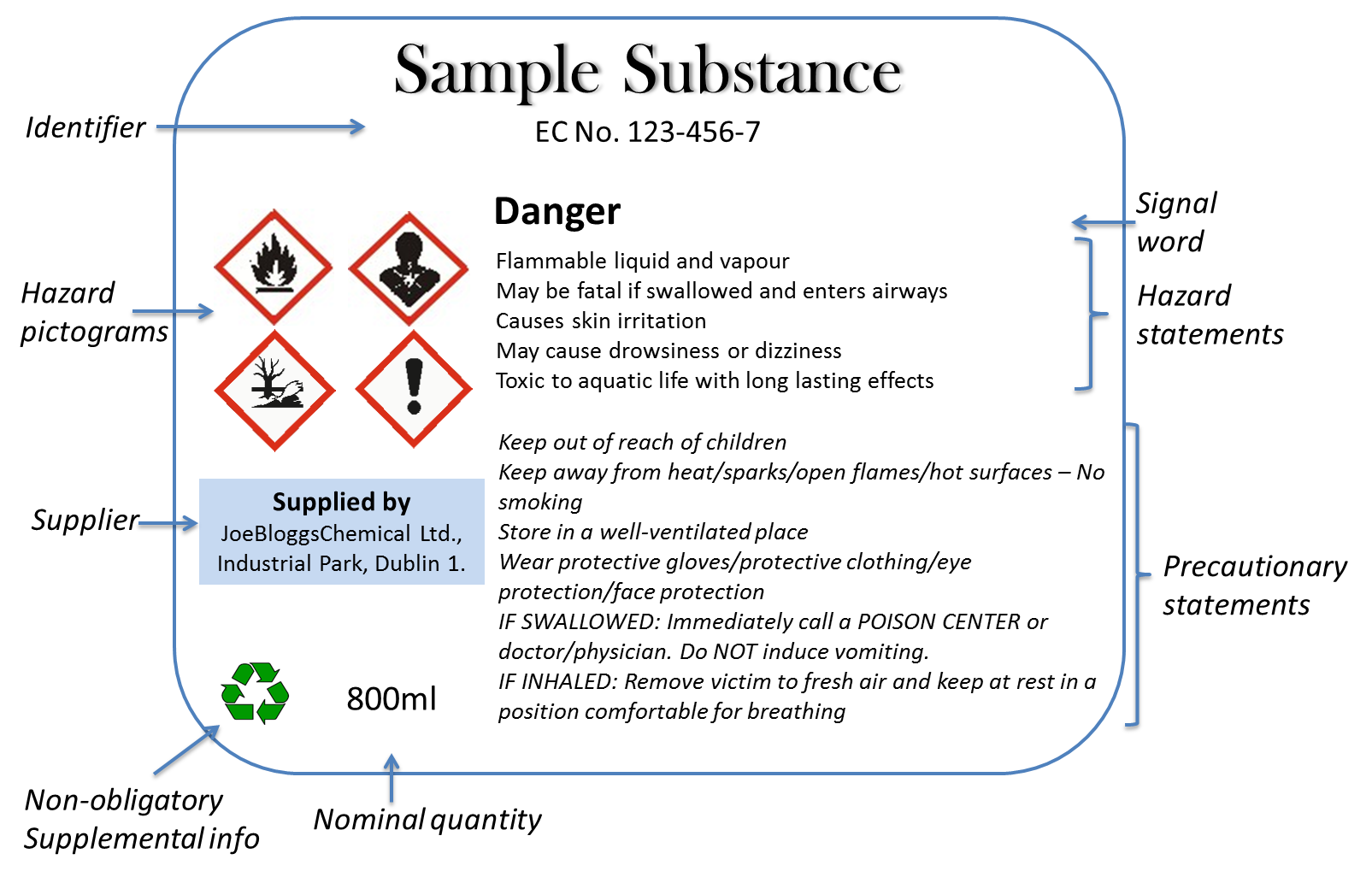
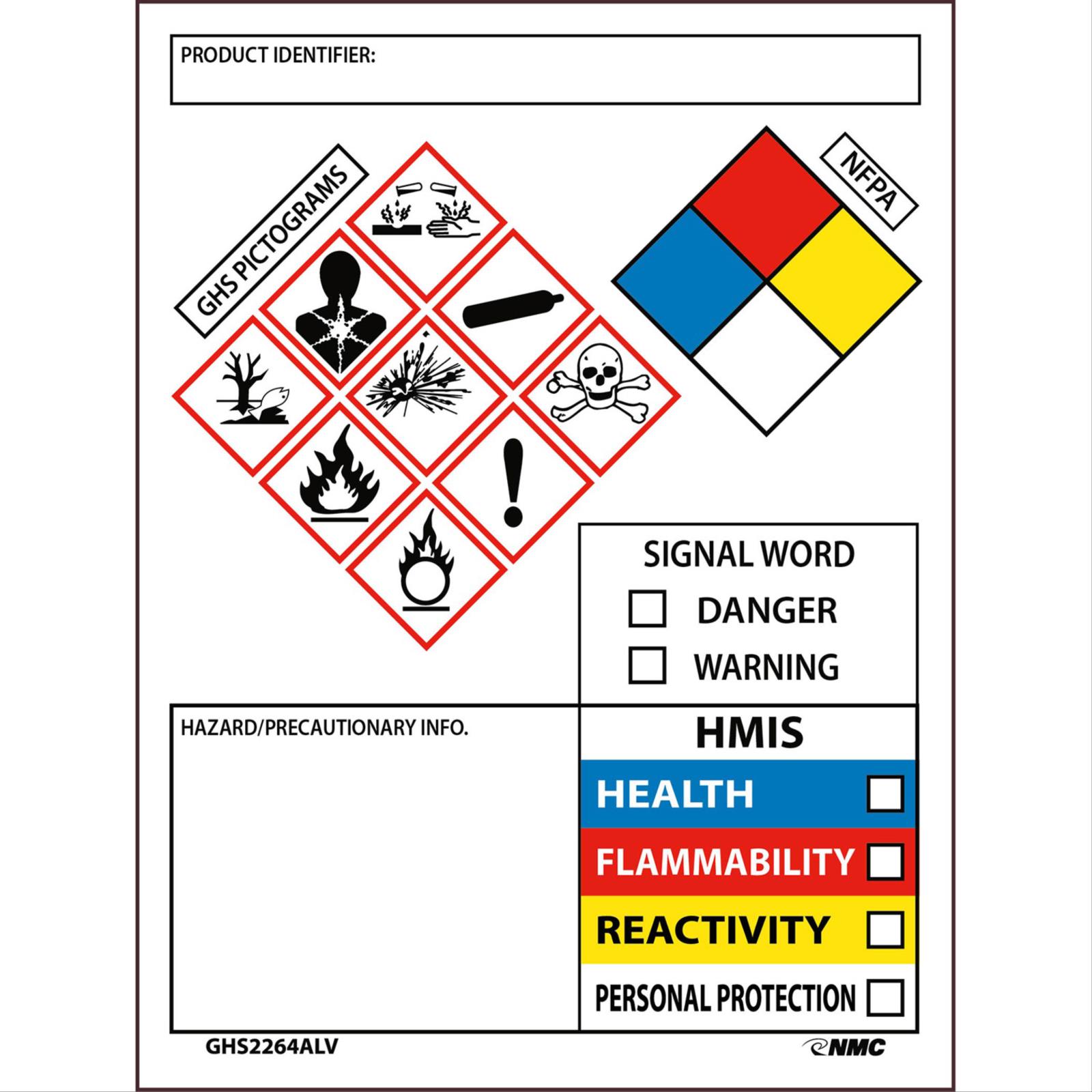
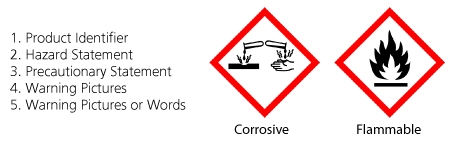
Labeling of Secondary Containers | Occupational Safety and Health ... Labeling of Secondary Containers Standard Number: 1910.1200 (f) (6) (ii) OSHA requirements are set by statute, standards and regulations. Our interpretation letters explain these requirements and how they apply to particular circumstances, but they cannot create additional employer obligations. GHS Labeling Requirements: The Definitive Guide [2021 Update ... - Luminer What Are GHS Label Requirements? Product Identifier GHS Signal Words Hazard Statements Precautionary Statements Name, Address, Telephone Number Pictograms Health Hazard Flame Exclamation Mark Gas Cylinder Corrosion Exploding Bomb Flame Over Circle Skull and Crossbones Environment Supplemental Information Container Types Primary Containers
GHS Label Requirements: The Complete GHS Labeling Guide - TapeManBlue Primary and secondary containers Both primary and secondary containers need to be labeled in order to be considered GHS compliant. Primary containers are typically the large barrels, boxes, or drums that come directly from a manufacturer. Any label already placed on a primary container should not be altered or removed.

Secondary label requirements
eCFR :: 21 CFR Part 201 -- Labeling The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented according to the schedule specified in § 201.56(c), except for the requirement in paragraph (c)(18) of this section to reprint any FDA-approved patient labeling at the end of prescription drug labeling or accompany the ... Secondary Containers and Service Containers for Pesticides As it isn't required that a secondary container label be submitted, there are no requirements per se. EPA will review them on a case-by-case basis and would be likely to accept them if: The EPA-approved master label includes directions for diluting the product. The secondary container label is submitted as part of the master label. Quality System Regulation Labeling Requirements | FDA Labeling specifications are: engineering drawing and/or artwork for each label, appropriate inspection or control procedures, and appropriate procedures for attaching the labels. All...
Secondary label requirements. [ ] Bovine spongiform encephalopathy (BSE) Chemicals in food - maximum residue limits. Food and medicine regulation. Food incidents. Food labelling. Food recalls Rules for Proper Secondary Container Labeling - HSI Secondary Container Label Requirements Employers must make sure each container of hazardous chemicals in the workplace is labeled, tagged, or marked with either of the following: All the specific information for the labels on shipped containers. › reference › formsForms | GSA Forms Library page. FORMS LIBRARY ASSISTANCE: Forms@GSA.gov LATEST UPDATES. GSA 1217 - Lessor's Annual Cost Statement - Renewed - 1/6/2023. GSA 1364WH - Proposal to Lease Space (Warehouse Request) - Renewed - 1/6/2023 Secondary Container Labels 101: HazCom and WHMIS - ERA Environmental Secondary Container Labels 101: HazCom and WHMIS Secondary Container Labels 101: HazCom and WHMIS This post was written by Alexandra McDougall Alexandra is a GHS Regulatory Specialist at ERA and head of the SDS Management Team.
The Basics of Secondary Container Labeling - FinishMaster The Basics of Secondary Container Labeling. Labeling is required by governing agencies including the Environmental Protection Agency (EPA), the Occupational Safety and Health Administration (OSHA), the Department of Transportation (DOT), and the National Fire Protection Association (NFPA). OSHA and EPA inspect and fine for unlabeled containers. historycooperative.org › mason-dixon-lineThe Mason-Dixon Line: What? Where? And why is it important? Sep 30, 2019 · The British men in the business of colonizing the North American continent were so sure they “owned whatever land they land on” (yes, that’s from Pocahontas), they established new colonies by simply drawing lines on a map. Then, everyone living in the now-claimed territory, became a part of an English colony. A map of the British Home page - SSERC Professional Learning. SSERC offers a vast portfolio of professional learning (PL) programmes for STEM educators in Scotland. Our curricular based PL is offered to everyone: childminders, early years workers, primary and secondary staff as well as lecturers, technicians and those who work with young people in non-formal settings such as youth workers and in the CLD sector. | The UK children's charity | NSPCC The NSPCC is the UK's leading children's charity, preventing abuse and helping those affected to recover. Help us be here for children. Please donate now.
› publications › p970Publication 970 (2021), Tax Benefits for Education | Internal ... Even though the same term, such as qualified education expenses, is used to label a basic component of many of the education benefits, the same expenses aren't necessarily allowed for each benefit. For example, the cost of room and board is a qualified education expense for the qualified tuition program, but not for the education savings bond ... What Information Is Required On Secondary Container Labels? - XO Safety While chemical shipping containers must have the full GHS label, OSHA provides employers with a lot of flexibility to create their own secondary container labeling systems. OSHA Requirements for Secondary Container Labels OSHA requires secondary container labels to have the full GHS label, or: OSHA clarifies secondary container labeling guidelines for employers - AAHA Initially it seemed as though veterinarians would be required to display GHS labels on all chemical products within the hospital, meaning hospitals would have to change their secondary labeling procedures. The latest guidance from OSHA reveals that the organization has not changed the general requirements for workplace labeling. When it comes ... Download secondary chemical container labels | EHS You may choose from three designs: Secondary Container Label 1 Secondary Container Label 2 Secondary Container Label 3 Exemption: Secondary labels are not required when the container is under the direct control of the person who transferred or prepared the solution and all of it will be consumed during the same work shift.
Device Labeling | FDA - U.S. Food and Drug Administration Section 201 (m) defines 'labeling' as: 'all labels and other written, printed, or graphic matter. (1) upon any article or any of its containers or wrappers, or. (2) accompanying such article' at ...
Labeling secondary containers - JJKellerSafety.com What information is required on secondary container labels? If the chemical is going to be used only "in house," then the container is to be labeled in accordance with 1910.1200 (f) (6) . Label it with the product identifier, words, pictures, symbols, or a combination thereof.
support.microsoft.com › en-us › officeTrack changes in Word - support.microsoft.com The Highlight Changes options on the Tools > Track Changes menu (Highlight changes on screen, Highlight changes in printed document) and the options on the Review tab pop-up menu (Final Showing Markup, Final, Original Showing Markup, Original) are not saved settings.
Secondary Container Label Requirements | HCL Labels Labeling Requirements for Secondary Containers These secondary containers are required to be labeled with a GHS chemical label, given if any of the following events occur: -The material is not used within the work shift of the individual who makes the transfer. -The worker who made the transfer leaves the work area.
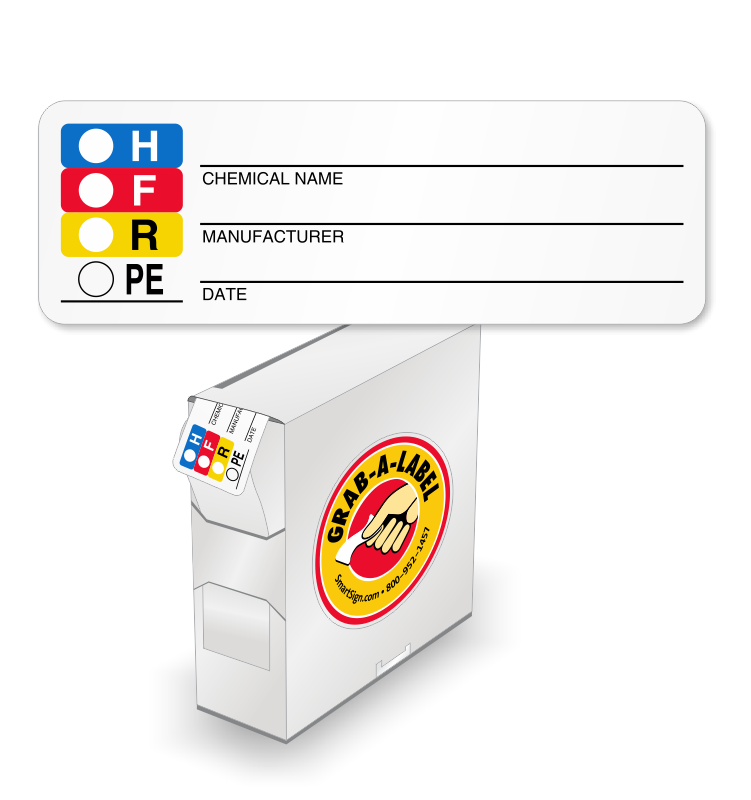
Secondary Labels Secondary Labels. Screen printed 32 oz spray bottles; Meets OSHA secondary labeling requirements; More permanent option to secondary label; Help with brand promotion; Sold in any quantity; Also available as private label . Recent Posts. Baker's Dozen Promotion;
EU - Labeling/Marking Requirements - International Trade Administration CE Mark. CE marking is probably the most widely used and recognized marking required by the European Union. Found in all "New Approach" legislation with a few exceptions, the placement of the CE mark on a product serves as the manufacturer's declaration that the item meets all EU regulatory requirements (typically related to safety, health, energy efficiency, or environmental concerns ...
What Required Information Must GHS Labels Include? - MPC GHS-compliant labels contain six main elements.Note that these requirements apply to primary containers (which includes the containers received from the manufacturer), but not specifically to secondary containers (such as smaller jars or spray bottles that hold chemicals transferred from the primary container).
What Is a Secondary Label and What Makes It OSHA Compliant? Steps to Take to Make Sure the Secondary Label is OSHA Compliant OSHA requires that you label hazardous chemicals using six indicators. These indicators communicate the physical, health, and environmental hazards associated with a particular chemical. Some of the steps that will increase your compliance with these guidelines include:
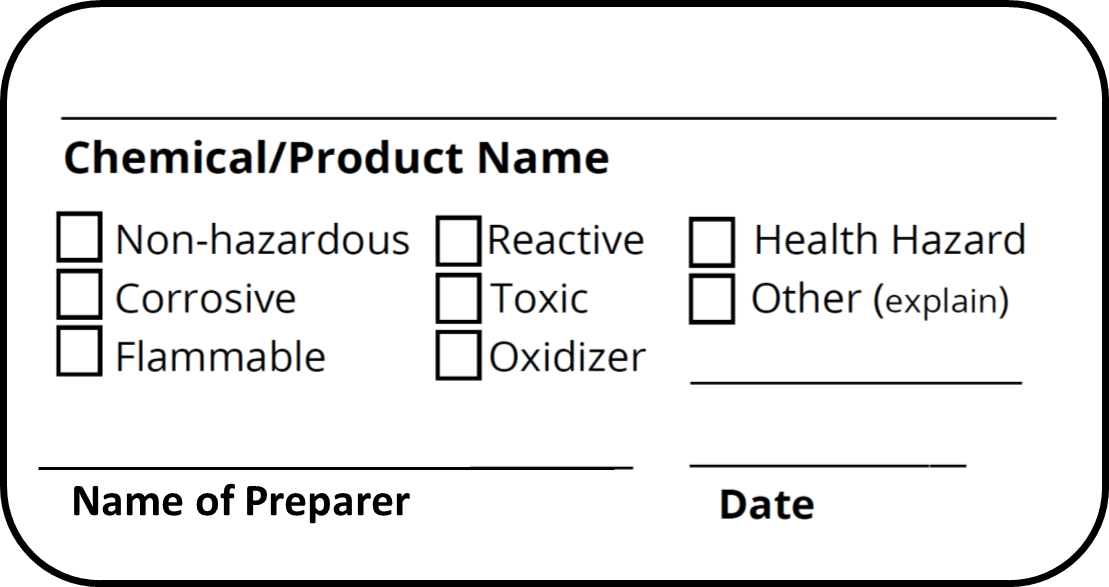
PDF Guidelines for Labeling Secondary Containers - UGA Non-Hazardous Substance Labeling Secondary containers for non-hazardous substances (e.g., saline, feed, water) must be labeled with the name of the substance, and dated if perishable or sterile. Perishable items must be labeled with an expiration date*. When a container is dated, the date must be clearly distinguished as either a fill date,
Chemical Container Labels | EHS - University of Washington Secondary container labels are not required if both of the following apply: The reagent, stock solution and chemicals mixed for use are under the direct control of the person who transferred or prepared it, and The container will be emptied during that person's work shift. Secondary chemical container label templates
Secondary Container Labeling Requirements - Texas Label Printers Secondary Container Labeling Requirements When dealing with hazardous chemicals, special identification known as secondary container labels are required for the transference of smaller amounts from the original container to a secondary one. Any other containers such as flasks, beakers, and smaller bottles are considered secondary containers.
4 Quick Tips to Help You Ace OSHA Secondary Container Labeling Information Secondary Container Labels Must Contain OSHA labeling requirements for secondary containers (that do notqualify for exemption) are outlined in HazCom standard 1910.1200(f)(6)(ii) and summarized listed below. Product Name/Identifier. This should match the product identifier on the safety data sheet. General Hazard Statement.
Labeling of secondary containers in laboratories under the Hazard ... The Laboratory standard requires that labels on incoming containers of hazardous chemicals not be removed or defaced, 1910.1450 (h) (1) (i), but does not have a specific labeling requirement for secondary containers of hazardous chemicals in a covered laboratory.
Secondary Containers | Environmental Health, Safety and Emergency ... When Do Secondary Containers Have to Be Labeled? Except for a few cases, secondary containers must be labeled. IF IN DOUBT, LABEL IT! One common case where you do not have to label a secondary container is if the container is portable and will be used immediately by the person who transferred the chemical into that container.
Compliance FAQs: Packaging and Labeling in the US | NIST There are many regulations, depending on the product, with which a product's label or markings must be in compliance before being sold in the United States. Labeling requirements related to legal metrology (i.e., products and commodities sold in package form by weight, measure or count) must comply with The Fair Packaging and Labeling Act ...
Labeling Requirements | US EPA Labeling Requirements EPA reviews the product label as part of the licensing/registration process for pesticides. The label on a pesticide package or container and the accompanying instructions are a key part of pesticide regulation.
Quality System Regulation Labeling Requirements | FDA Labeling specifications are: engineering drawing and/or artwork for each label, appropriate inspection or control procedures, and appropriate procedures for attaching the labels. All...
Secondary Containers and Service Containers for Pesticides As it isn't required that a secondary container label be submitted, there are no requirements per se. EPA will review them on a case-by-case basis and would be likely to accept them if: The EPA-approved master label includes directions for diluting the product. The secondary container label is submitted as part of the master label.
eCFR :: 21 CFR Part 201 -- Labeling The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented according to the schedule specified in § 201.56(c), except for the requirement in paragraph (c)(18) of this section to reprint any FDA-approved patient labeling at the end of prescription drug labeling or accompany the ...























Post a Comment for "39 secondary label requirements"